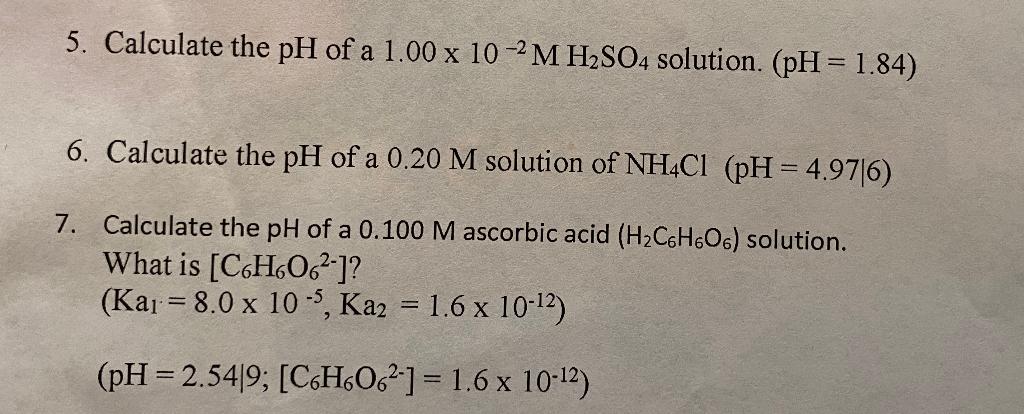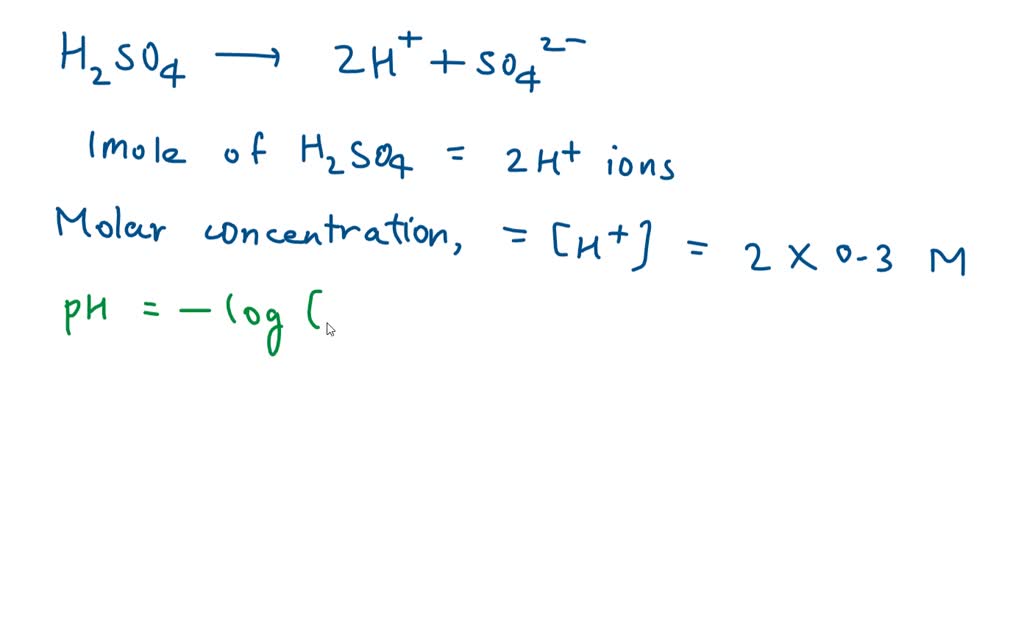![SOLVED: Calculate the pH of a 0.1wt% H2SO4 SULFURIC ACID solution. You can assume that the density of the solution is that of water. Must use ph = -log[h+]. Please explain and SOLVED: Calculate the pH of a 0.1wt% H2SO4 SULFURIC ACID solution. You can assume that the density of the solution is that of water. Must use ph = -log[h+]. Please explain and](https://cdn.numerade.com/ask_previews/fb910f96-3dc9-421e-9ac5-8d5a0aaf4d70_large.jpg)
SOLVED: Calculate the pH of a 0.1wt% H2SO4 SULFURIC ACID solution. You can assume that the density of the solution is that of water. Must use ph = -log[h+]. Please explain and
![SOLVED: Find the pH of a 0.0065 M sulfuric acid solution. Find [SO42?] of a 0.0065 M sulfuric acid solution. Answer in M SOLVED: Find the pH of a 0.0065 M sulfuric acid solution. Find [SO42?] of a 0.0065 M sulfuric acid solution. Answer in M](https://cdn.numerade.com/ask_previews/5be48cb9-2b9b-4861-a0b2-0ce3c9e0c22f_large.jpg)
SOLVED: Find the pH of a 0.0065 M sulfuric acid solution. Find [SO42?] of a 0.0065 M sulfuric acid solution. Answer in M

Curve for pH as a function of sulfuric acid concentration based on the... | Download Scientific Diagram
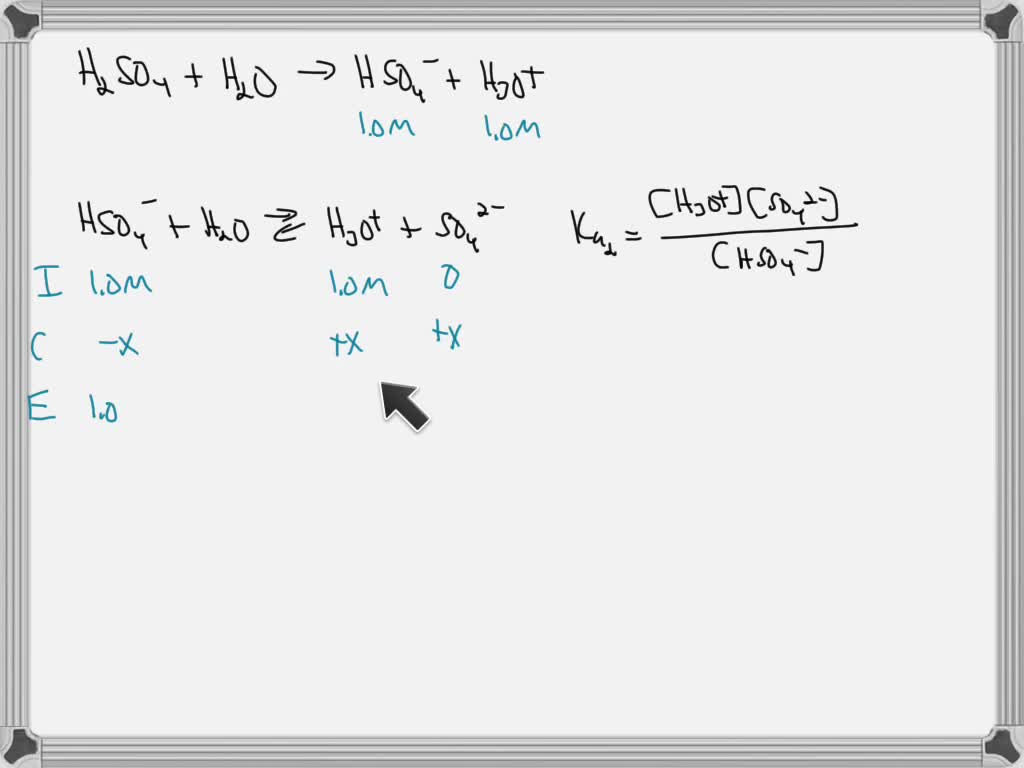
![Chapter [ ] Acids and Bases Equilibria. Arrhenius (or Classical) Acid-Base Definition An acid is a substance that contains hydrogen and dissociates. - ppt download Chapter [ ] Acids and Bases Equilibria. Arrhenius (or Classical) Acid-Base Definition An acid is a substance that contains hydrogen and dissociates. - ppt download](https://images.slideplayer.com/23/6591516/slides/slide_39.jpg)
Chapter [ ] Acids and Bases Equilibria. Arrhenius (or Classical) Acid-Base Definition An acid is a substance that contains hydrogen and dissociates. - ppt download
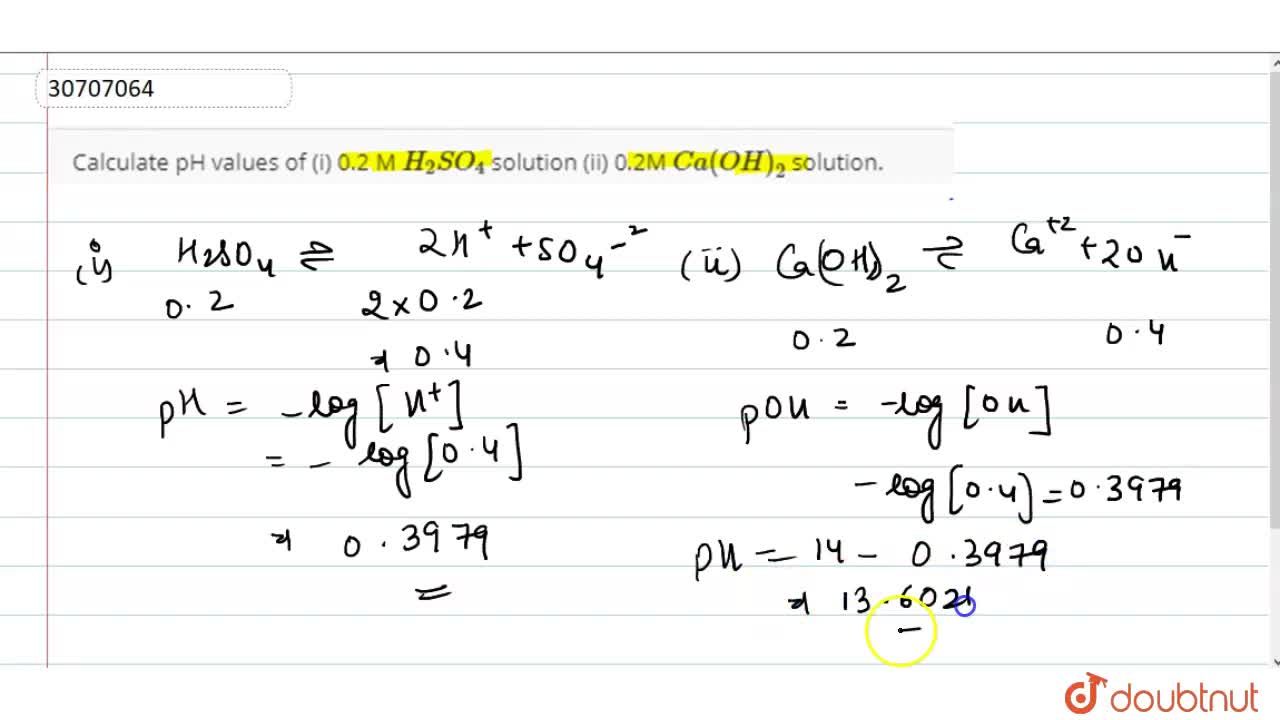
![SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and](https://cdn.numerade.com/ask_previews/5338532b-72ac-4610-a96e-d01f0328e5e1_large.jpg)
SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and

Spectroscopic Measurement of pH in Aqueous Sulfuric Acid and Ammonia from Sub- to Supercritical Conditions | Industrial & Engineering Chemistry Research