Calculate the volume occupied by 8.8g of CO2 at 31.1C and 1 bar pressure. R= 0.083 bar dm3 K-1 mol-1 - YouTube

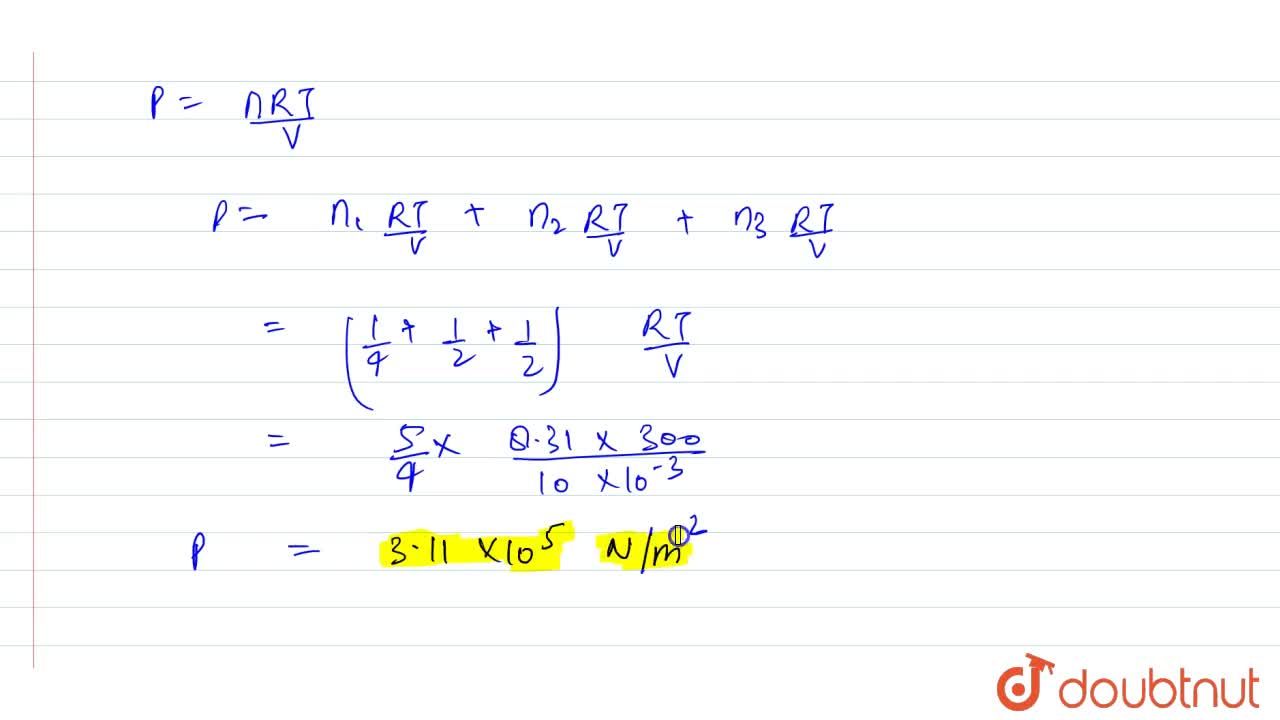
8g oxygen, 14 g nitrogen and 22 g carbon dioxide are mixed in a container of volume 4 l. Find out the pressure of the gas mixture at 27^(@)C. Given R =

Convert 22 g of carbon dioxide `(CO_(2))` into moles. (Atomic masses : `C = 12 u, O = 16 u`) - YouTube

Calculate the amount of carbon dioxide that could be produced when (i) 1 mole of carbon is burnt in air.(ii) 1 mole of carbon is burnt in 16 g of dioxygen.(iii) 2

what volume of `CO_2` will be liberated at STP if 12 g of carbon is burnt in excess of oxygen ? - YouTube

ACT Practice Questions.docx - ACT Practice Questions A scientist studying hemoglobin investigated the impact of temperature and carbon dioxide CO2 | Course Hero

CO 2 results of entire MIS 11, including end of MIS 12. Dome C CO 2... | Download Scientific Diagram

A Step toward the Quantification of Noncovalent Interactions in Large Biological Systems: The Independent Gradient Model-Extremely Localized Molecular Orbital Approach | Journal of Chemical Information and Modeling

PDF) Oxygen isotope anomaly in tropospheric CO2 and implications for CO2 residence time in the atmosphere and gross primary productivity

Metal–CO2 Electrochemistry: From CO2 Recycling to Energy Storage - Wang - 2021 - Advanced Energy Materials - Wiley Online Library